Your Location:Home > Products > Veterinary APls > Oclacitinib Maleate
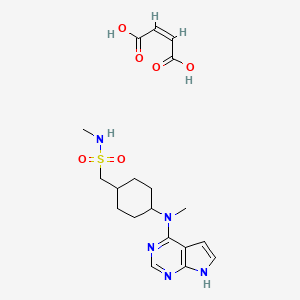


CasNo: 1208319-27-0
MF: C19H27N5O6S
Oclacitinib is labeled to treat atopic dermatitis and itchiness (pruritus) caused by allergies in dogs, though it has also been used to reduce the itchiness and dermatitis caused by flea infestations. Oclacitinib is a Janus kinase (JAK) inhibitor used for control of pruritus in dogs associated with allergic dermatitis and atopic dermatitis. Oclacitinib has a unique mechanism of action that acts to inhibit proinflammatory cytokines. Liaoning Pharmaceutical Innovation Co.,Ltd. has a R&D center, a pilot workshop and a GMP production base. It integrates scientific research, pilot testing and production, and is committed to providing high-quality pharmaceutical chemicals and contract processing services to the world's pharmaceutical companies. As a research-first and customer-centered enterprise, we have always been unremittingly improving the technological innovation system and prioritizing technological innovation. We also use advanced and complete production facilities and a complete quality assurance system to ensure the safe production of high-quality products. . Positive and innovative ideas, dedicated and dedicated employees, and sufficient and reasonable resource allocation are the firm cornerstones that promote the gradual development and growth of Fuyin Technology.
The invention discloses a preparation me...
The invention discloses a preparation me...
The present invention relates to solid s...
Described herein are improved processes ...
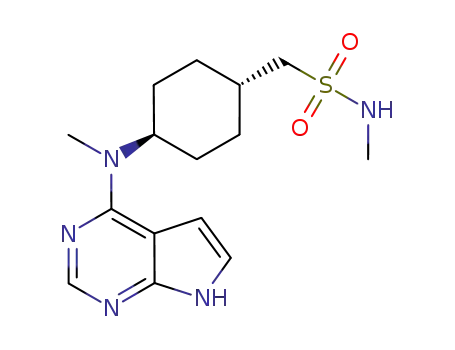
Oclacitinib

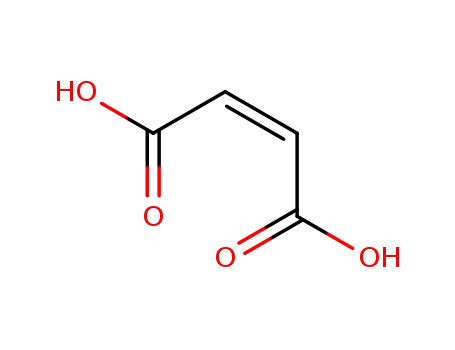
maleic acid

![N-methyl-1-{trans-4-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclohexyl}methanesulfonamide maleate](/upload/2024/11/a31749bf-e19d-4a2e-be4d-c832af1187e7.png)
N-methyl-1-{trans-4-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclohexyl}methanesulfonamide maleate
| Conditions | Yield |
|---|---|
|
With pyrographite; In ethanol; at 78 - 80 ℃;
|
95.6% |
|
In water; at 37 - 60 ℃; Temperature;
|
|
|
In water; butan-1-ol; at 20 ℃; for 18h;
|
253.0 g |
![N-methyl-1-{trans-4-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclohexyl}methanesulfonamide](/upload/2024/11/5f9f15e1-db58-4480-924e-ad47779c2a27.png)
N-methyl-1-{trans-4-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclohexyl}methanesulfonamide

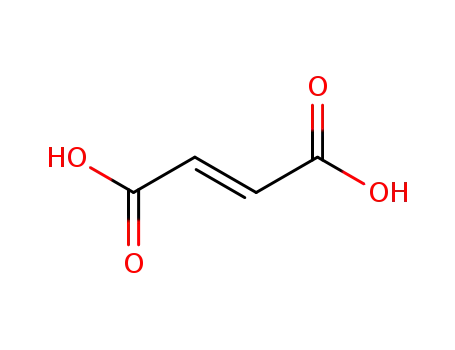
(2E)-but-2-enedioic acid

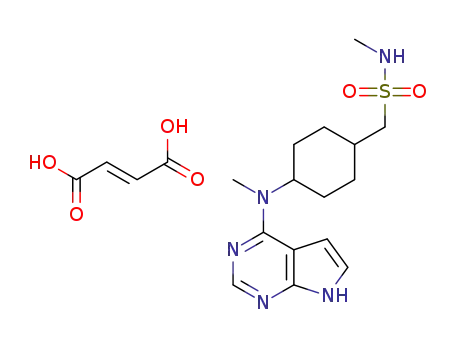
oclacitinib maleate
| Conditions | Yield |
|---|---|
|
With pyrographite; In ethanol; at 78 - 80 ℃;
|
95.5% |

maleic acid
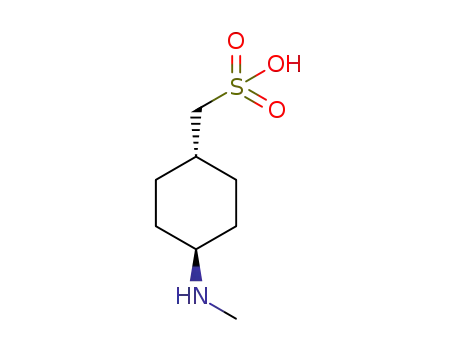
trans-4-((methylamino)cyclohexyl)methanesulfonic acid
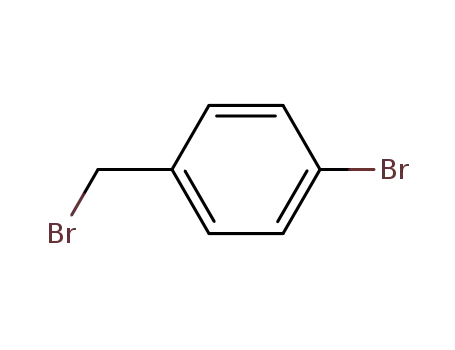
1-bromomethyl-4-bromobenzene
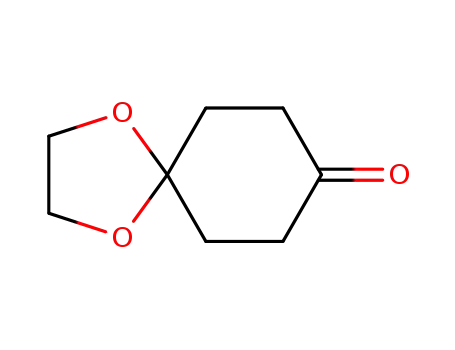
cyclohexanedione monoethylene ketal