Your Location:Home > Products > Human APls > Fulvestrant
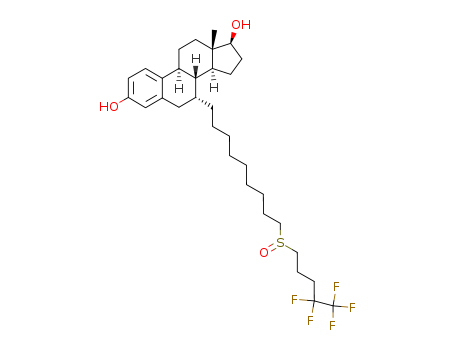


CasNo: 129453-61-8
MF: C32H47F5O3S
Appearance: white powder
|
Indications and Usage |
Fulvestrant is a muscle injection drug developed by the company AstraZeneca and is suitable for treating postmenopausal women with estrogen receptor-positive metastasized breast cancer whose condition continued to worsen despite antiestrogen treatment. Fulvestrant is the only antiestrogen drug that can be widely clinically used following unsuccessful tamoxifen treatment. This drug is a type of endocrine therapy, so it will not cause any adverse effects commonly seen in chemotherapy, giving it relatively good patient compliance. Multiple clinical trials have found that 250mg Fulvestrant is effective and consistently safe as a second line of treatment for advanced breast cancer. |
|
Mechanisms of Action |
Many breast cancer cells contain estrogen receptors (ER), so estrogen stimulates breast cancer growth. Fulvestrant is a steroid estrogen receptor antagonist, and its chemical structure is similar to estradiol, except that its 7α position contains a linking group. Fulvestrant is a 17β-estradiol alkylamine analogue, and it binds with, prevents, and decreases ER to inhibit the estrogen signal transduction pathway. It binds competitively with ER, has a similar affinity with ER as estrogen, and inhibits gene activation stimulated by estrogen, thus affecting necessary estrogen-related processes in cell circulation. Its fulvastans have a similar affinity with ER as estrogen and is 100 times that of tamoxifen. |
|
Pharmacokinetics |
Fulvestrant has a relatively poor oral bioavailability, so it is commonly injected into the muscle with lipids as excipients. In an open, random and multicenter study on postmenopausal women with advanced breast cancer, one 5ml or 2 2.5ml dosages containing 250mg were injected, and their pharmacokinetics and poisonous side effects did not differ greatly, while its blood concentration was dose-dependent and had individual differences. In the 7-day treatment period, serum LH, FSH or SBHG levels did not change significantly. This drug does not pass through the blood-brain barrier and will not cause side effects such as vasomotor symptoms. |
|
Adverse reactions |
Fulvestrant causes relatively fewer side effects, including brief vaginal bleeding, body odor change, and sleepwalking. There have not been any reports of effects such as vaginal dryness, weight gain, blood clotting abnormalities, thrombus formation and libido change, and characteristics such as facial flushing and sweating are not affected. A small-scale stage III clinical trial on 19 women with metastasized breast cancer who used this drug showed that its clinical efficacy was 67% and there were no serious safety issues. It showed that continuous monthly injections were crucial and that it was well-tolerated, with only slight swelling and paint at injection site, while facial flushing, uterine lining thickness, sex hormone binding globulin levels, follicle stimulating hormones levels, and luteinizing hormone levels all showed no change. |
|
Biological Activity |
A high affinity estrogen receptor antagonist (IC 50 = 0.29 nM), devoid of any partial agonism both in vitro and in vivo . Also high affinity agonist at the membrane estrogen receptor GPR30. |
|
Biochem/physiol Actions |
Fulvestrant (ICI 182,780) is a selective estrogen receptor down-regulator (SERD). Fulvestrant is a high affinity estrogen receptor antagonist. IC50 = 0.29 nM. Fulvestrant is the first "pure" antiestrogen with no agonistic activity both in vitro and in vivo. |
|
Side effects |
Side effects appear to be minimal and include several GI symptoms , headache, and hot flashes . There is no clinical evidence of uterine stimulation or laboratory evidence of stimulation of endometrial carcinoma models. Fulvestrant should not be adm inistered to women who are pregnant, who are taking antic oagulants, or who have thrombocytopenia. |
|
Synthesis |
Fulvestrant is administered as a once a month i. m. injection. Several routes for the synthesis of fulvestrant (12) were published. One of the best routes is depicted in the scheme. The conjugate addition of Grignard reagent derived from bromide 130 with dienone 129 gave adduct 131 as a mixture of 7α- and 7β-isomers in a ratio of 2.5:1 in 90-95% yield. Aromatization of the A-ring with copper bromide/lithium bromide in acetic acid followed by hydrolysis of the ester group provided diol 132 in 80-85% yield. Oxidation of the side chain from sulfite to sulfone followed by crystallization provided fulvestrant (12) in 30% overall yield from dienone 129. |
|
Drug interactions |
Potentially hazardous interactions with other drugs None known |
|
Metabolism |
The metabolism of fulvestrant has not been fully evaluated, but involves combinations of a number of possible biotransformation pathways analogous to those of endogenous steroids. Identified metabolites (includes 17-ketone, sulphone, 3-sulphate, 3- and 17-glucuronide metabolites) are either less active or exhibit similar activity to fulvestrant in anti-oestrogen models. Fulvestrant is eliminated mainly in metabolised form. The major route of excretion is via the faeces. |
|
Definition |
ChEBI: A 3-hydroxy steroid that is 17beta-estradiol in which the 7alpha hydrogen has been replaced by a nonyl group in which one of the hydrogens of the terminal methyl has been replaced by a (4,4,5,5,5-pentafluoropentyl)sulfinyl group. An estrogen receptor antagonist, it is used in the treatment of breast cancer. |
|
Brand name |
Faslodex (AstraZeneca). |
| Manufacturer | Liaoning Pharmaceutical Innovation Co.,Ltd. was registered on September 16, 2021, with a registered capital of 12 million. It is located in Panjin Fine Chemical Industrial Park, Liaoning. It is an enterprise integrating the development, research and production of raw materials and intermediate processes. Liaoning Pharmaceutical Innovation Co.,Ltd. has a R&D center, a pilot workshop and a GMP production base. It integrates scientific research, pilot testing and production, and is committed to providing high-quality pharmaceutical chemicals and contract processing services to the world's pharmaceutical companies. |
InChI:InChI=1/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22?,26-,27+,28+,29-,30+,41?/m1/s1
-
Fulvestrant (Faslodex) was synthesized i...
The invention belongs to the field of ph...
The invention relates to a method for pr...
The present invention provides a method ...
![(+)-(7α)-[9-(4,4,5,5,5-pentafluoropentylsulfanyl)nonyl]estra-1,3,5(10)-triene-3,17β-diol](/upload/2024/11/b06d0030-ffc7-4f00-9a81-2cd28823bca9.png)
(+)-(7α)-[9-(4,4,5,5,5-pentafluoropentylsulfanyl)nonyl]estra-1,3,5(10)-triene-3,17β-diol

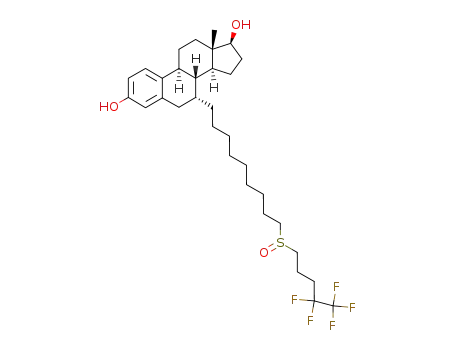
fulvestrant
| Conditions | Yield |
|---|---|
|
With dihydrogen peroxide; acetic acid; In water; ethyl acetate; at 40 ℃; for 4h; Inert atmosphere; Schlenk technique;
|
95% |
|
(+)-(7α)-[9-(4,4,5,5,5-pentafluoropentylsulfanyl)nonyl]estra-1,3,5(10)-triene-3,17β-diol; With fipronilβ-cyclodextrin; In water; acetone; at 20 - 60 ℃; for 0.5h;
With N-Bromosuccinimide; In water; acetone; at 40 ℃; for 10h; Temperature;
|
87% |
|
With dihydrogen peroxide; acetic acid; In water; ethyl acetate; for 6 - 8h;
|
85% |
|
With dihydrogen peroxide; acetic acid; In water; ethyl acetate;
|
85% |
|
With dihydrogen peroxide; acetic acid; In water; ethyl acetate; at 0 - 25 ℃; Solvent; Reagent/catalyst; Temperature;
|
85% |
|
With dihydrogen peroxide; acetic acid; In water; ethyl acetate; at 0 - 25 ℃; Solvent; Reagent/catalyst; Temperature;
|
85% |
|
With dihydrogen peroxide; acetic acid; In ethyl acetate; at 20 ℃; for 16h;
|
80% |
|
With peracetic acid; acetic acid; In ethyl acetate; at 20 - 25 ℃; for 2h;
|
76% |
|
With sodium periodate; In methanol; water; at 20 ℃; for 24h; Inert atmosphere;
|
75% |
|
With dihydrogen peroxide; acetic acid; In ethyl acetate; at 20 - 30 ℃; for 8h; Large scale;
|
53% |
|
With sodium periodate; In tetrahydrofuran; methanol; water; at 5 - 20 ℃; Product distribution / selectivity;
|
|
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 0 - 5 ℃; for 2h;
|
2.35 g |
|
With dihydrogen peroxide; acetic acid; In water; ethyl acetate;
|
|
|
With sodium iodate; In methanol; water; at 20 ℃; for 24h; Reagent/catalyst; Solvent; Temperature; Cooling with ice;
|
|
|
(+)-(7α)-[9-(4,4,5,5,5-pentafluoropentylsulfanyl)nonyl]estra-1,3,5(10)-triene-3,17β-diol; With dihydrogen peroxide; acetic acid; In water; ethyl acetate; for 12h; pH=7;
With sodium sulfite; In water; for 0.5h;
With sodium hydroxide; In water; ethyl acetate; for 0.5h; Reagent/catalyst; Temperature;
|
3.5 g |
|
With dihydrogen peroxide; acetic acid; In ethyl acetate; at 20 - 25 ℃;
|
0.7 g |
![7β-[9-(4,4,5,5,5-pentafluoropentylsulfanyl)nonyl]estra-1,3,5-trien-3,17β-diol](/upload/2024/11/692ebb2e-5676-49b3-a9c6-65d35bd60fc3.png)
7β-[9-(4,4,5,5,5-pentafluoropentylsulfanyl)nonyl]estra-1,3,5-trien-3,17β-diol


fulvestrant
| Conditions | Yield |
|---|---|
|
With dihydrogen peroxide; acetic acid; In ethyl acetate;
|
82% |
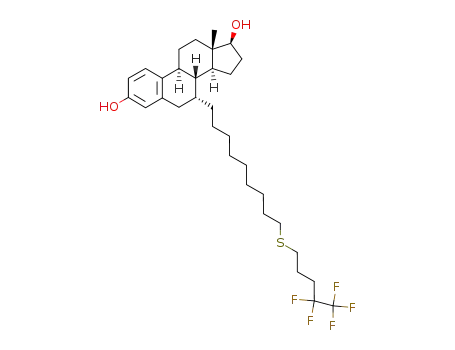
(+)-(7α)-[9-(4,4,5,5,5-pentafluoropentylsulfanyl)nonyl]estra-1,3,5(10)-triene-3,17β-diol
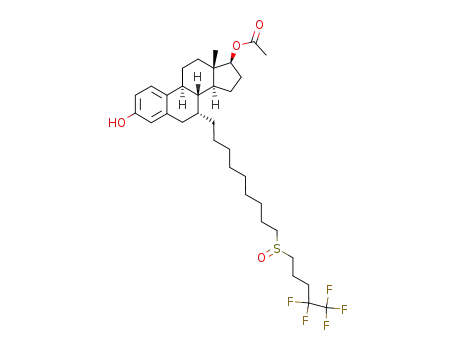
fulvestrant 17β-acetate
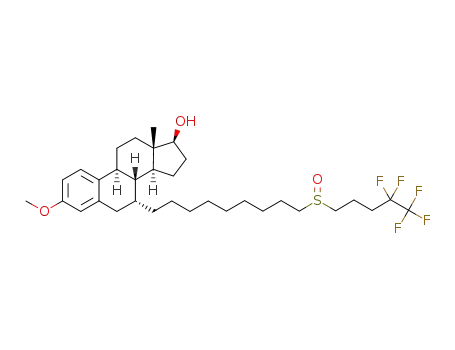
(7R,13S)-3-methoxy-13-methyl-7-(9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl)-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-17-ol
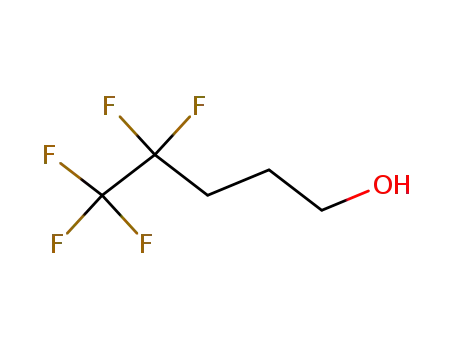
4,4,5,5,5-pentafluorpentan-1-ol
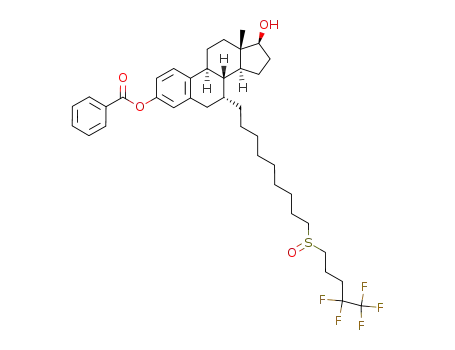
fulvestrant 3-benzoate

fulvestrant 17β-acetate
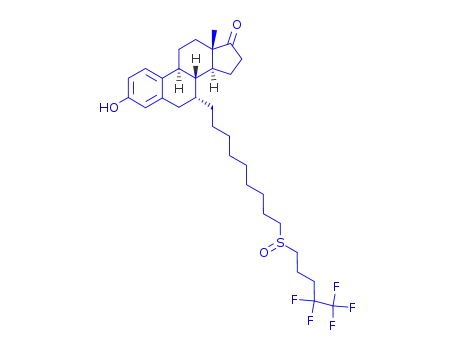
ICI 182,780-17-ketone
6-{3-benzoyloxy-13-methyl-7-[9-(4,4,5,5,5-pentafluoro-pentane-1-sulfinyl)-nonyl]-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-17-yloxy}-3,4,5-tris-isobutyryloxy-tetrahydro-pyran-2-carboxylic acid methyl ester